Abstract
Introduction: The Myeloma Genome Project (MGP) characterized the genomic landscape of patients with newly diagnosed multiple myeloma (NDMM) (Walker BA, et al. Blood 2018; 132[6]:587-597). Using a multi-omics unsupervised clustering approach, 12 molecularly-defined disease segments were identified (Ortiz M, et al. Blood 2018; 132[suppl 1]:3165). Here, we performed experimental validation of CDC28 Protein Kinase Regulatory Subunit 1B (CSK1B) that was identified as a putative target from the disease segment with poorest clinical outcome. CKS1B was selected for in-depth validation due to their role in cell cycle pathways associated with high-risk disease, biological mechanisms of chromosome 1q amplification and druggability.
Methods: Association of CKS1B with outcomes was analyzed in NDMM patients, across relapses and with clinical outcome datasets from MGP and Mayo clinic. Inducible shRNAs of CKS1B and bromodomain containing protein 4 (BRD4, a member of the BET [bromodomain and extra terminal domain] family) were generated in MM cell lines. BRD4 and Aiolos ChIP-seq datasets were analyzed for binding on CKS1B gene. BRD4 inhibitors JQ1 and CC-90010 were utilized for inhibition studies in MM cell lines.
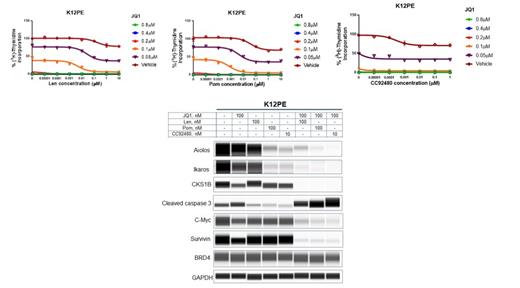
Results: Higher expression of CKS1B was associated with significantly poorer PFS, OS, disease severity and relapse. Knock-down of CKS1B in MM cells led to a significant decrease in proliferation (P<0.001) and enhanced apoptosis in MM cell lines. BRD4-ChIP sequencing studies revealed that the expression of CKS1B was regulated by super-enhancer (SE) associated elements. As expected, two BRD4 inhibitors, JQ1 and CC-90010 and inducible BRD4 shRNAs downregulated the expression of CKS1B resulting in decreased proliferation, cell cycle arrest and apoptosis in MM cell lines. Furthermore, MM cell lines harboring chromosome 1q gain/amp showed higher sensitivity to BRD4 inhibition compared to cell lines with normal 1q copy number. Mechanistic studies revealed that BRD4inh and BRD4 shRNAs downregulated the expression of Aiolos and Ikaros in MM cell lines. Interestingly, Aiolos ChIP-sequencing studies demonstrated the binding of Aiolos at the transcriptional start sites of CKS1B with the transcriptional activation mark. The immunomodulatory agent (IMiD ®) pomalidomide (Pom) transcriptionally downregulated CKS1B in Pom-sensitive cells downstream of Aiolos, Ikaros degradation. Based on these mechanisms, IMiD agents, lenalidomide, Pom and the novel Cereblon E3 ligase modulating degrader (CELMoD ®) agent CC-92480 in combination with BRD4inh promoted a synergistic decrease in proliferation, cell cycle arrest and increase in apoptosis in both Pom-sensitive and -resistant cell lines. The combination of IMiD or novel CELMoD agent with BRD4inh also promoted deeper downregulation of CKS1B, Aiolos, Ikaros, c-Myc and survivin proteins with enhanced levels of apoptotic marker cleaved Caspase 3 as compared to single agents alone.
Conclusions: In summary, we have identified CKS1B as a key target associated with poor outcome in MM patients. Translational studies suggest a profound downregulation of CKS1B and key pro-survival effector proteins following combination treatment with BRD4inh and IMiD agents/novel CELMoD agents resulting in synergistic anti-tumor effects. These data provide rationale for testing these agents in the clinic for high-risk and IMiD-relapsed patients.
Figure: Changes in cell proliferation and protein levels of key signaling mediators were studied in K12PE cell line treated with increasing doses of Lenalidomide, Pomalidomide and CC-92480 in combination with JQ1.
Ahsan: BMS: Current Employment, Current equity holder in publicly-traded company. Polonskaia: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hsu: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bjorklund: BMS: Current Employment, Current equity holder in publicly-traded company. Ortiz Estevez: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Towfic: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bahlis: Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Genentech: Consultancy; Pfizer: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria. Pourdehnad: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties: No royalty. Flynt: BMS: Current Employment, Current equity holder in publicly-traded company. Ahsan: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Thakurta: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal